Abstract
Recent studies have shown that, in contrast to typical proliferating lymphocytes, alloreactive T (allo-T) cells can be specifically killed by apoptosis through compounds that lead to generation of intracellular reactive oxygen species. Nuclear factor erythroid-derived 2-like 2 (Nrf2) is an ubiquitously expressed transcription factor well-known for its role in regulating the cellular redox pathway, but little is known about its involvement in T-cell biology. In this study, we examined the contribution of Nrf2 to allo-T cells in the context of an allogeneic hematopoietic cell transplant (allo-HCT).
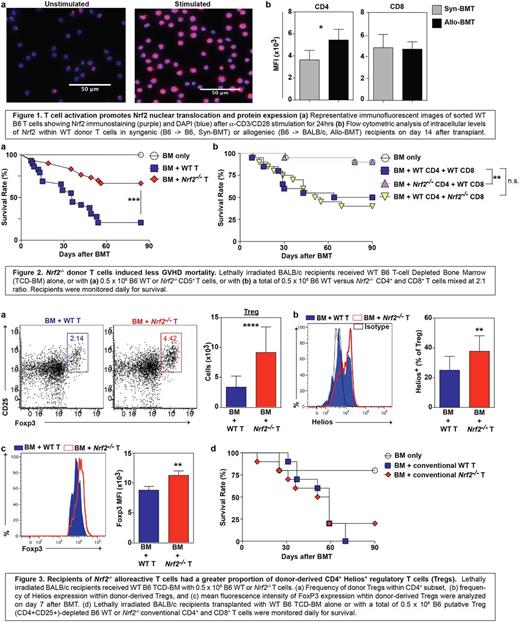
To study the role of Nrf2 in T cell function, we first assessed the expression pattern of Nrf2 in activated T cells. We found that total cellular and nuclear Nrf2 expression in T cells were significantly increased 24 hours after in vitro α-CD3/CD28 stimulation (p<0.001), as well as in vivo in donor allo-T cells on day 14 in a well-established MHC-disparate murine allo-HSC model (C57BL/6→BALB/c) (p<0.05) (Fig 1). We next examined the effects of Nrf2 deficiency on T cell alloreactivity by transplanting WT or Nrf2-/- donor T cells in MHC-disparate (C57BL/6→BALB/c), as well as MHC-matched, minor antigen mismatched (C57BL/6→LP) murine models. In both models, allo-HCT recipients of Nrf2-/- donor T cells had significantly less acute graft-versus-host disease (GVHD)-induced mortality (p<0.01), morbidity (p<0.001), and intestinal pathology (p<0.05) (Fig 2a). To test if Nrf2 differentially regulates CD4+ versus CD8+ T cell function, we mixed and matched WT versus Nrf2-/- CD4+ and CD8+ T cells for transplant. Compared to allo-HCT recipients of donor WT CD4+: WT CD8+ T cells, we observed similar GVHD mortality in allo-HCT recipients of donor WT CD4+: Nrf2-/- CD8+ T cells, while we saw reduced GVHD mortality in allo-HCT recipients of donor Nrf2-/- CD4+: WT CD8+ T cells (p<0.01) (Fig 2b). These data support the notion that Nrf2 predominately regulates donor CD4+ and is dispensable for CD8+ T cells during allo-activation and the development of GVHD.
We assessed if Nrf2 could affect polarization of donor CD4 T cells after allo-HCT, and found a two-fold increase in the proportion and absolute numbers of CD25+Foxp3+ regulatory T cells (Tregs) in the spleens of donor Nrf2-/- T cell allo-HCT recipients on day 7 post transplant (p<0.001) (Fig 3a). We next examined if Nrf2-deficiency results in greater induction of Tregs (iTregs) or stabilization of natural Tregs (nTregs). While we found an equal fraction of WT and Nrf2-/- naïve CD4+ T cells induced towards Tregs in vitro, we saw a significantly greater percentage of donor Nrf2-/- Tregs expressing Helios, a marker for thymus-derived nTregs, compared to donor WT Tregs in allo-HCT recipients on day 7 (p<0.01) (Fig 3b). Recent studies have suggested that Helios is required to stabilize the expression of FoxP3, and thus, the abundance and inhibitory activity of Tregs in inflammatory settings. In keeping with this notion, we discovered an increase in the cellular FoxP3 protein expression within donor Nrf2-/- Tregs in allo-HCT recipients (p<0.01) (Fig 3c). To further elucidate the biological significance of Nrf2 function on nTregs, we removed CD4+CD25+ putative Tregs from the donor inoculum and transplanted only conventional T cells (Fig 3d). We found that the survival advantage with donor Nrf2-/- T cells was abolished when Tregs were depleted from the donor graft, suggesting that Nrf2 expression in donor T cells negatively regulates nTregs during allo-activation, most likely through downregulation of Helios and FoxP3 expression.
Finally, to determine if Nrf2 was critical for T-cell mediated GVT activities, we challenged allo-HCT recipients with A20 (B-cell lymphoma) cells. Using bioluminescent imaging, we found that Nrf2-/- allo-T cells were equally efficient in clearing tumor burdens, and along with their mitigated GVHD activities, resulting in a significantly improved survival outcome.
Taken together, we identified a novel role of Nrf2 in CD4+ T cells in the context of allo-HCT. Nrf2 negatively regulated CD4+ nTreg through Helios downregulation during GVHD, but appeared to be dispensable in CD8+ mediated antitumor responses, further highlighting the possibility of separating GVT from GVHD activities. Our findings suggest that targeted manipulation of Nrf2 activities in allo-T cells may offer a useful clinical strategy to mitigate GVHD while preserving GVT responses.
Jenq: Seres: Research Funding. van den Brink: PureTech Health: Consultancy; Seres: Research Funding; Jazz Pharmaceuticals: Consultancy; Therakos Institute: Other: Speaking engagement.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal